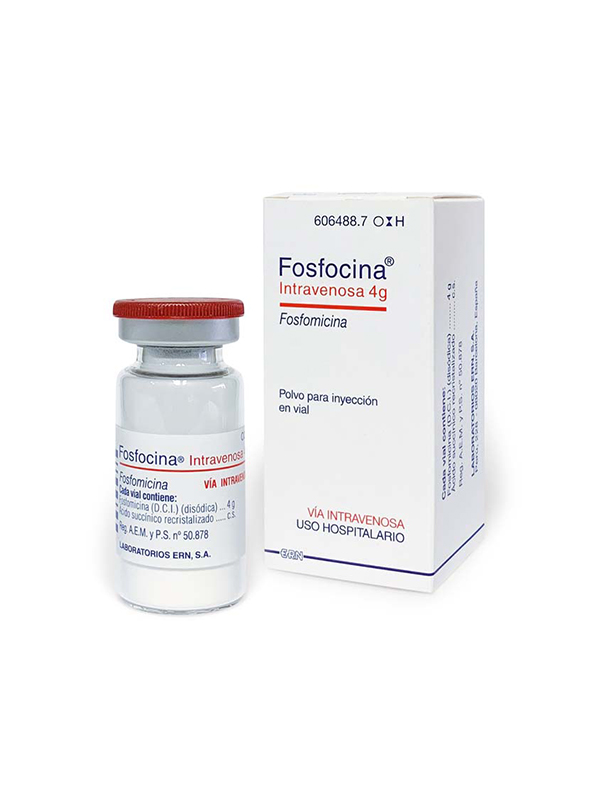
Fosfocina intravenous 4 g
Fosfocina intravenous 4 g
Fosfomycin
Antibiotic indicated for the treatment of complicated or severe urinary, dermatological, gynaecological, respiratory, musculoskeletal, surgical infections, septicaemias, endocarditis and meningitis caused by sensitive microorganisms.
Prescription drug
Not funded (National Health System)
Clinical container
- 759662.2 (1 vial)