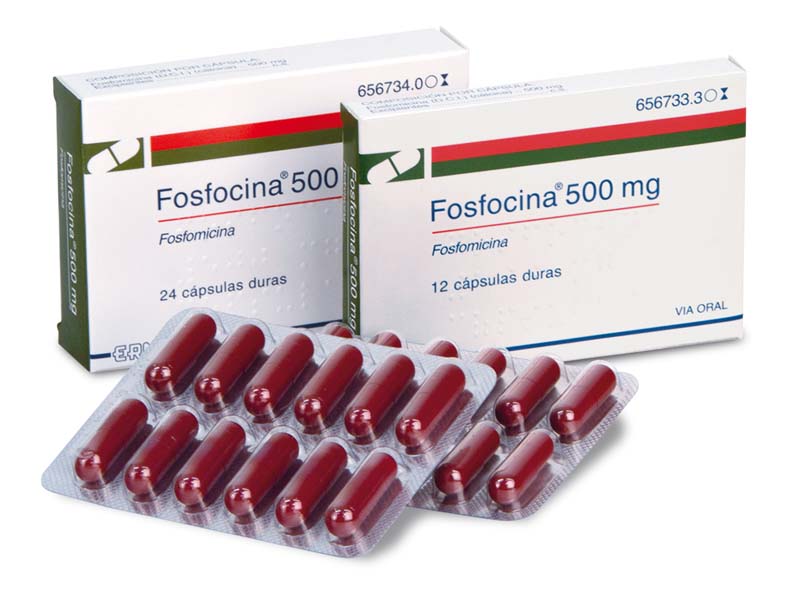
Fosfocina, 500 capsules
Fosfocina, 500 capsules
Fosfomycin
Antibiotic indicated for the treatment of uncomplicated urinary tract infections, gastrointestinal tract infections and dermatological infections caused by sensitive microorganisms.
Prescription drug
Not funded (National Health System)
Clinical container
- 604728.6 (500 capsules)
-
Gluten-free